Thermogravimetric Analysis with Differential Scanning Calorimetry
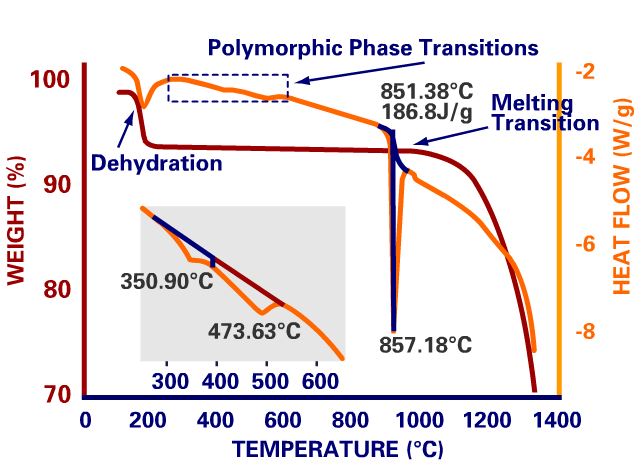
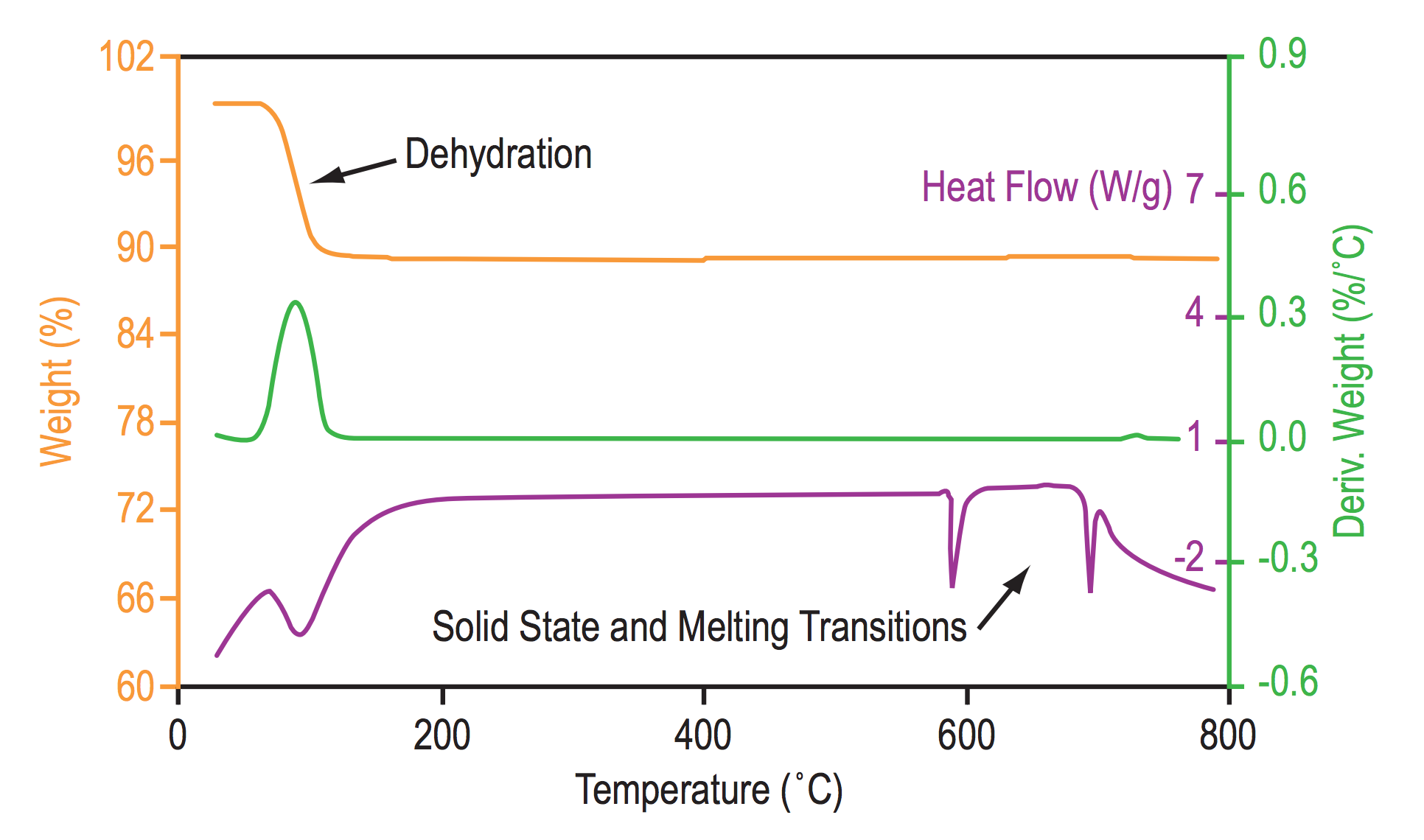
Thermogravimetric analysis (TGA) is a method of thermal analysis in which changes in physical and chemical properties of materials are measured as a function of increasing temperature (with constant heating rate), or as a function of time (with constant temperature and/or constant mass loss).
TGA can provide information about physical phenomena, such as:
- second-order phase transitions (including vaporization, sublimation, absorption, adsorption, and desorption)
- chemisorption
- desolation (especially dehydration),
- decomposition
- solid-gas reactions (e.g., oxidation or reduction)
Differential scanning calorimetry (DSC) is a thermo-analytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time.
DSC can provide information about physical phenomena, such as:
- Melting Point/Melting Range
- Heat Capacity
- Crystallization
- Glass Transition
- Thermal Stability
- Decomposition Temperature
- Oxidative Induction Times (OIT)
- Purity